A previous blog explained the difference in understanding calibration and validation. However, people often question how these two terms compare to verification and testing, which again are individual processes in their own right. In this month’s blog, we will define the four key terms, calibration, validation, verification and testing to provide laboratory users with a better understanding.
Understanding calibration, validation, verification and testing
In laboratory, clinical and medical device environments, quality and reliability are essential. Yet, the terms calibration, validation, verification and testing are often misunderstood or used interchangeably. To comply with international standards such as ISO/IEC 17025, ISO 15189, ISO 13485 and ISO 9001, it is crucial to distinguish clearly between these concepts. This article offers a concise explanation, helping quality professionals and laboratory managers ensure clarity and compliance:
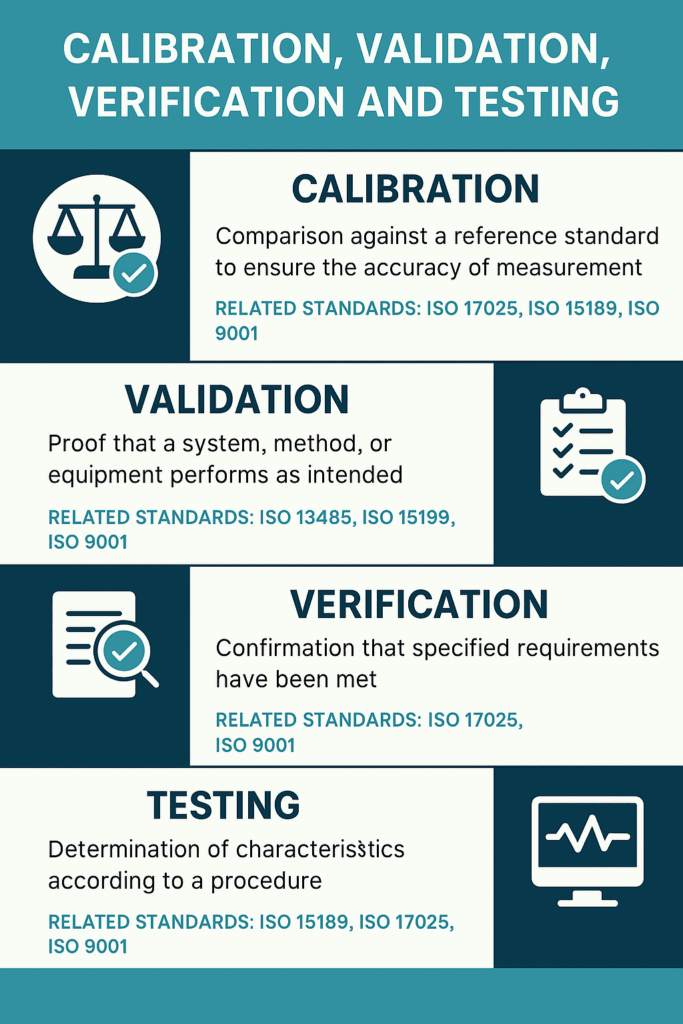
Calibration
Calibration is defined as the comparison of a measurement instrument against a traceable reference standard to determine and, if necessary, correct its accuracy. According to ISO/IEC 17025 and ISO 15189, calibration must be traceable to national or international standards, and the associated measurement uncertainty must be known and documented.
For example, calibrating a laboratory thermometer ensures its readings are within acceptable limits compared to a certified temperature source. Calibration does not validate the instrument for any specific use—it simply confirms measurement accuracy under defined conditions. In ISO 9001 terms, calibration supports the control of monitoring and measuring resources (Clause 7.1.5), helping ensure product conformity.
Validation
Validation refers to the process of proving that a system, method or equipment performs as intended, reliably and consistently, under actual conditions of use. As emphasised in ISO 13485 (for medical devices) and ISO 15189 (for medical laboratories), validation is essential for processes where the outcome cannot be fully verified through subsequent inspection or testing.
Validation typically includes:
- Installation Qualification (IQ) – ensuring the system is installed correctly;
- Operational Qualification (OQ) – confirming it functions within set parameters;
- Performance Qualification (PQ) – verifying consistent performance in real-world use.
For instance, validating an autoclave involves demonstrating that it consistently sterilises according to protocol across a variety of loads.
Verification
Verification is the confirmation that specified requirements have been met, based on objective evidence. It is generally more limited in scope than validation and is often a one-time activity. According to ISO 9001, verification supports product and process control, while ISO 17025 applies it to confirm the reliability of testing and calibration methods. An example would be verifying that a new spectrophotometer passes all manufacturer-specified operational checks upon installation. Verification does not necessarily involve calibration or long-term performance assessment.
Testing
Testing involves determining the characteristics of a product, material or system by applying a defined procedure. It can be part of validation or verification, and is often used to generate objective evidence in support of both.
In ISO 15189, testing is central to medical laboratory services, ensuring patient safety and result reliability. In ISO 17025, it supports technical competence by demonstrating that methods and equipment yield accurate results.
Summary
The below infographic neatly summarises the differences between the four terms:
Final Thoughts
Understanding the nuances between calibration, validation, verification and testing is essential for regulatory compliance, quality management and risk mitigation. Organisations operating in laboratory, medical or industrial sectors should embed these principles within their quality systems, aligning with the expectations of ISO 9001, ISO 15189, ISO 13485 and ISO/IEC 17025 to ensure reliability, safety, and trust in their operations.
How Henderson Biomedical can help
Henderson Biomedical can calibrate your laboratory equipment to ISO 17025 standards. Our understanding of calibration regularly audited by UKAS and we are on hand to help with your needs. For more information, please contact us using our online form or call us on 020 8663 4610.
Image by Freepik